Resident Program - Case of the Month
February 2020 - Presented by Dr. Alexander Ladenheim (Mentored by Dr. Maija Kiuru)
Diagnosis
Gram stain and PAS were unremarkable. A Fite stain showed numerous acid-fast bacilli in the superficial and deep dermis, within nerves (Figure 4), and in endothelial cells.
Figure 4 Fite, high power. Nerve demonstrating numerous organisms and globi (arrow).
The diagnosis rendered was multibacillary leprosy with associated vasculitis, consistent with erythema nodosum leprosum (ENL; type 2 reaction). The specimen was sent out to the National Hansen’s Disease Program who agreed with the diagnosis and performed PCR testing of the specimen, which was positive for Mycobacterium leprae.
Discussion
Hansen’s disease, or leprosy, affects over 220,000 people worldwide,1 mainly in Brazil, India, Indonesia, Myanmar, and Nigeria.2 It is caused by Mycobacterium leprae, an obligate intracellular, microaerophilic, rod-shaped, fastidious organism. It has not been successfully cultured on artificial media and so is generally grown and studied in various animal models.3 Like other pathogenic Mycobacteria, M. leprae is phagocytosed by macrophages but evades destruction in phagosomes. It disseminates in the blood, and because it replicates well at 32-34oC, it exhibits tropism for the cutaneous tissues and extremities.4 M. leprae is also unique among bacteria in exhibiting neurotropism for reasons that are poorly understood.3 The organism has no particular virulence factors beyond its cell wall, which helps it survive after phagocytosis.4
The manifestations of infection with M. leprae are dependent on host immune response, which in turn appears to be in large part genetically determined. The host innate immune response likely determines initial susceptibility to infection. If the bacteria is able to evade host innate immune defenses, the degree of cell-mediated immune response and the degree of hypersensitivity reaction to M. leprae antigens determines the clinical manifestations of the disease.3
Patients with a robust cell-mediated response develop tuberculoid and borderline lesions. Patients develop one or more well demarcated, erythematous, flat lesions that become indurated and can ulcerate. Affected areas develop sensory and motor neuropathy. Histologically, biopsies of lesions show well-developed granulomatous inflammation, and acid-fast staining shows very sparse organisms and so are termed paucibacillary lesions.4 Because organisms are sparse, PCR detection of bacterial DNA is an important adjunct to diagnosis in paucibacillary lesions in particular.1
On the other hand, lepromatous lesions develop in patients with little to no resistance to the bacteria. These lesions are poorly demarcated and numerous, diffuse, and tend to be nodular. Biopsies reflect more diffuse dermal infiltration by macrophages, and acid-fast staining will demonstrate large numbers of organisms (multibacillary lesions); at the extreme end, clumps of bacteria (globi) can be seen.4
Hansen disease is treated with combination antibiotic therapy in response to strains of the bacteria that developed resistance to the original monotherapy, dapsone. Today, in paucibacillary disease, dapsone is combined with rifampicin. In the multibacillary form, clofazimine is also added. For treatment purposes, the WHO recognizes that tissue diagnosis may not be available or adequate in many parts of the world, and the classification is often made clinically. Patients having 5 or fewer skin lesions are considered to have paucibacillary disease, and patients with more than 5 lesions or with a skin biopsy positive for organisms to have multibacillary disease. The duration of therapy is usually 1-2 years,2 requiring intense efforts on the part of patients and clinicians to complete the regimen.
Over the course of their illness, about half of patients experience acute flares of the illness called reactions which may occur in treated or untreated disease. Type 1 reactions occur in patients with tuberculoid and borderline lesions. Existing lesions become erythematous and indurated over the course of weeks with exacerbation of neuropathy. Type 2 reactions, also known as erythema nodosum leprosum (ENL), affect patients with lepromatous lesions. In this form, patients suddenly develop new tender, erythematous, nodular lesions, often with accompanying systemic inflammatory signs like fever and malaise.3 This reaction may be mediated by an immune complex formation caused by the formation of pathologic antibodies to M. leprae antigens. ENL is characterized by an intense, mixed inflammatory infiltrate in the dermis and subcutaneous fat with acute vasculitis, and organisms can be seen in endothelial cells. On the extreme end, a very rare, life-threatening reaction, the Lucio phenomenon, is a severe, necrotizing vasculitis primarily seen in Mexico and Central America. It occurs in patients with untreated or undertreated leprosy and presents with irregularly shaped, angulated purpuric patches and plaques that ulcerate. Histologically, findings of the Lucio phenomenon are similar to ENL but typically also show thrombosis of blood vessels.1 Recognition of these acute reactions is important, because corticosteroids and other immunosuppressive agents can be effective in curbing both type 1 and type 2 reactions.3
References
- Grayson, W. “Chapter 18: Infectious diseases of the skin.” In Calonje JE, ed. McKee’s Pathology of the Skin. 4th ed. Edinburgh: Mosby Ltd; 2012;825-831.
- World Health Organization. WHO Model Prescribing Information: Drugs Used in Leprosy. Geneva, Switzerland: WHO; 1998. http://apps.who.int/medicinedocs/en/d/Jh2988e/. Accessed January 6, 2020.
- Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. “The Continuing Challenges of Leprosy.” Clinical Microbiology Reviews 2006 Apr;19(2):338-381. doi:10.1128/CMR.19.2.338–381.2006.
- McAdam AJ, Milner DA, Sharpe AH. “Chapter 8: Infectious Diseases.” In Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Saunders; 2015:377-378.

 Meet our Residency Program Director
Meet our Residency Program Director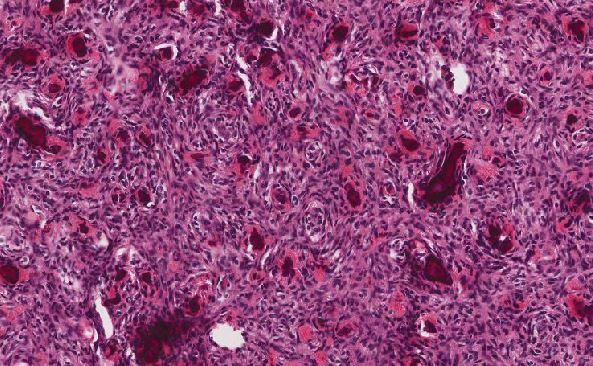
 LeShelle May
LeShelle May Chancellor Gary May
Chancellor Gary May