State stem cell agency, Shriners Children’s award $15 million to UC Davis CuRe trial
Funding will support clinical trial testing stem cell treatment for spina bifida
A team of UC Davis Health researchers, led by pioneering fetal surgeon Diana Farmer and bioengineer Aijun Wang, has been awarded nearly $15 million for groundbreaking work on a spina bifida treatment. The funding will allow the team to carry out phase 2 of a clinical trial that tests the first stem cell treatment for the birth defect. Spina bifida, also known as myelomeningocele, occurs when spinal tissue in a fetus fails to fuse properly during the early stages of pregnancy.
Launched in the spring of 2021, the CuRe clinical Trial (Cellular Therapy for In Utero Repair of Myelomeningocele) tests a one-of-a-kind treatment that uses placenta-derived stem cells to improve outcomes for children with spina bifida. The treatment, a stem cell patch, is delivered directly to the spinal opening of the fetus while still developing in the mother’s womb.
In its board meeting yesterday, the California Institute for Regenerative Medicine (CIRM) approved an $8.9 million grant to fund the CuRe trial. Shriners Children’s matched this funding with a $5.9 million grant. The funding application reviewers voted unanimously that the CuRe study has exceptional merit and warrants funding.
“We want to thank CIRM for their support of this program and our partner Shriners Children’s for making this important work possible. Their funding will help us achieve our goal of using stem cells before birth to improve the ability to walk and have bowel and bladder control in patients born with spina bifida,” said Farmer, distinguished professor of surgery and principal investigator for the CuRe trial.
About spina bifida and the CuRe clinical trial
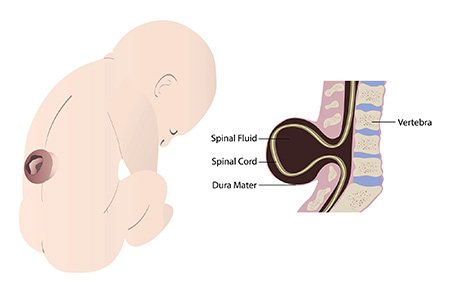
Spina bifida affects 1,500 to 2,000 children in the U.S. every year. Myelomeningocele is the most severe form of spina bifida. It results from incomplete closure of the spinal cord during gestation, which leaves a portion of the fetal spinal cord unprotected. The neuronal tissue damage to the spinal cord may lead to lifelong lower body paralysis and bowel and bladder dysfunction.
The Management of Myelomeningocele Study (MOMS) demonstrated that surgical fetal closure of the spinal cord defect in utero protected the previously exposed spinal cord and improved motor outcomes compared to repairing the defect after birth. The study established in-utero repair as the new standard of care of myelomeningocele.
The CuRe trial was developed to test whether placenta-derived mesenchymal stem cells can protect, repair and restore damaged spinal tissue beyond what surgery can accomplish alone.
In 2015, the project was funded through a CIRM Preclinical Developmental Award grant. Later, CIRM funded the research team with a $5.6 million Late Stage preclinical TRAN1 grant. The study was then approved by the U.S. Food and Drug Administration (FDA) in 2020 as a Phase 1/2a trial. In 2021, CIRM further funded the first phase of the trial with a $9 million grant.
During its first phase, the trial tested the safety of the treatment in six babies with spina bifida. The FDA and data monitoring board determined that the stem cell therapy is safe enough to proceed to the next phase of the trial.


“The CIRM awards have made it possible for our research team to generate clinical grade stem cells at the UC Davis Good Manufacturing Product (GMP) facility, carry out studies for the FDA investigational new drug approval, and complete Phase 1 of the trial,” Wang explained. Wang, who is an expert in stem cell biology and bioengineering, is the co-inventor of the placental-derived stem cell treatment and the study’s co-principal investigator with Farmer.
Farmer and Wang also serve as principal investigators with the Institute for Pediatric Regenerative Medicine (IPRM). IPRM is a collaborative initiative of the UC Davis School of Medicine and the Shriners Children's Northern California. They are also joined by a large team of innovators and researchers from the Wang Lab at the Center for Surgical Bioengineering.

Over the years, Shriners Children's has also funded several spina bifida-related studies led by Farmer and Wang. These projects include developing innovative tissue-engineered bony scaffold for fetal treatment of spinal bifida and new spina bifida disease models, such as the guinea pig and English bulldog.
The new combined award supplies critical funding to support phase 2a of the clinical trial. It will allow the researchers to evaluate the preliminary success of stem cell therapy in additional 29 patients, as approved by the FDA.
“We are very grateful and proud that we now have both CIRM and Shriners Children’s as our partners for the phase 2 portion of the CuRe trial! It is this important partnership that is making this groundbreaking work possible,” Wang said. “It is a testimony to the team science approach that our team has always been taking to conduct innovative research and solve unmet medical needs.”
Related Stories: